On April 11, 2018, the State Drug Administration issued five circulars in succession, first requiring Yunnan, Hunan and Anhui Food and Drug Administration to take back the "Drug GMP Certificate" for enterprises suspected of illegal production of drugs in their respective regions. Suspected illegal activities were investigated according to law. These include Yunnan Jinqi Pharmaceutical Co., Ltd., Hunan Baicao Pharmaceutical Co., Ltd., and Zhangzhou Jinglu Chinese Herbal Pieces Factory.
The next day, the State Drug Administration requested Jilin, Inner Mongolia, Sichuan and Shandong Food and Drug Administration to recover the relevant "Drug GMP Certificate" of the enterprises involved, and investigate the suspected illegal activities of the enterprise according to law. The companies involved include Baicheng Yizheng Pharmaceutical Co., Ltd., Chifeng Weikang Biochemical Pharmaceutical Co., Ltd., Sichuan Zining Chinese Herbal Pieces Co., Ltd., Shandong Kangning Pharmaceutical Co., Ltd. Shandong Linqing Huawei Pharmaceutical Co., Ltd., Inner Mongolia Mongolian Medicine Co., Ltd. And Kunming Pharmaceutical Group Co., Ltd.
The local drug supervision bureau will prevent the micro-duration, open the traceability mechanism, and crack down on enterprises that have problems in drug production.
On April 8, 2018, it was learned from the Anhui Food and Drug Administration that the bureau issued a notice to announce the results of the daily supervision and inspection of the pharmaceutical production and special drug operations in March 2018. Cangzhou Guoyitang Traditional Chinese Medicine Pieces Co., Ltd., Anhui Shunhetang The GMP certificates of three enterprises including Chinese Herbal Pieces Co., Ltd. and Anhui Daoyuantang Chinese Herbal Pieces Co., Ltd. were recovered.
On April 11, 2018, the Food and Drug Administration of Tongchuan City of Shaanxi Province issued the “Public Punishment Information Disclosure Form for March 2018â€. One of the two cases disclosed this time involved a pharmaceutical company, which was Shaanxi Xingshengde Pharmaceutical Co., Ltd. Production of inferior medicine acanthopanax. It was found that Shaanxi Xingshengde Pharmaceutical Co., Ltd., from December 15, 2016 to December 28, 2016, produced 87.3 kg of Acanthopanax senticosus with the batch number of 20161201, with a sample of 0.5 kg, and 0.8 kg for inspection and loss. The actual finished product was shipped out of the warehouse 86kg, sold 86kg, sold at a price of 19.7 yuan/kg, sold 39kg, totaling 768.30 yuan; sold at a price of 20.7 yuan/kg, sold 47kg, totaling 972.90 yuan.
On April 9, 2018, the Shanghai Food and Drug Administration issued the third phase of the drug supervision and sampling quality announcement in 2018. The bureau has implemented quality supervision and random inspection on the production, operation and use of pharmaceutical and pharmaceutical packaging materials in this Municipality, and will now announce the inspection of unqualified products (see table below).
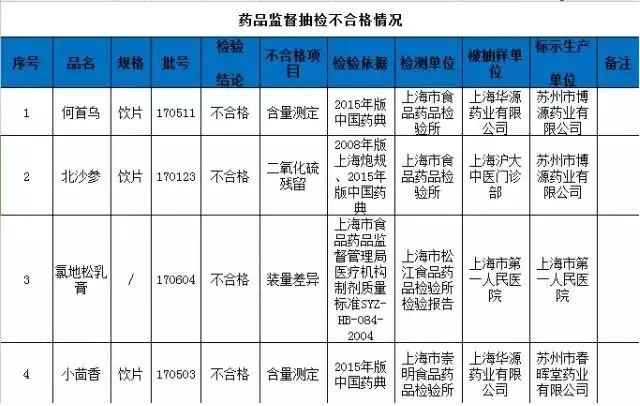
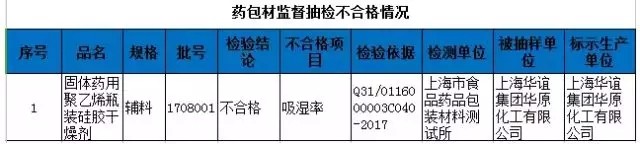
The drug dealers are not regulated, but they are punished, and the GSP is recovered.
On April 8, 2018, the Jiangdu District Market Supervision Bureau of Yangzhou City, Jiangsu Province continuously investigated and dealt with two cases of illegally purchased drugs. A pharmacy, illegally purchased drugs were not put on the shelves, hidden in the kitchen cabinets of the business premises, including 8 batches of captopril tablets and valsartan capsules, the value of 1180 yuan; another pharmacy, illegal purchase There are more medicines, including Naoxintong Capsules, Wenxin Granules, etc. There are 15 batches, which are directly sold on the shelves, with a value of 1730 yuan. Persons involved in the suspected case will be held liable.
On April 8, 2018, Dongguan City Food and Drug Administration of Guangdong Province cancelled 49 drug retail enterprises such as Guangdong Legend National Medicine Co., Ltd. Dongguan Nancheng Renai Branch and Dongguan City Herbal Medicine Chain Co., Ltd. Qiaotou Shishuikou Market Branch according to relevant laws and regulations. "Drug Business License".
On April 8, 2018, the Ningde Municipal Food and Drug Administration of Fujian Province, according to the relevant management measures and regulations, reclaimed the “Pharmaceutical Management Quality Management Certificate†of the 55th branch of Ningde City of Jianhui People Medical Chain Co., Ltd. according to law.
On April 10, 2018, Henan Nanyang City Food and Drug Administration published 2018 administrative punishment information, including Henan Guanbao Yuntong Pharmaceutical Co., Ltd., Nanyang Longtairen Pharmaceutical Co., Ltd., Nanyang Puqiang Pharmaceutical Co., Ltd. and other three drugs. The illegal facts involved in the enterprise are sales of inferior drugs; Nanyang Pingtangtang Parity Pharmacy Co., Ltd. is suspected of purchasing pharmaceuticals such as recombinant insulin glargine injection without having the qualification for pharmaceutical production and operation. Relevant departments have issued warnings, confiscation of illegal income, fines and other administrative penalties for these four companies.
On April 10, 2018, the Liaocheng Food and Drug Administration of Shandong Province, in accordance with the Drug Administration Law of the People's Republic of China and its implementation regulations and the Measures for the Administration of Pharmaceutical Business Licenses, applied for registration and agreed to write off Liaocheng Hongzhuoda. Pharmacy Co., Ltd. Fuping Sixth Store, Liaocheng Yimintang Da Yaodian Chain Co., Ltd., the fourteenth chain store, Donga County Longgongtang Pharmacy Co., Ltd., the first branch, Guanxian Kangjia Pharmacy, Liaocheng Dongchang In the "Drug Business License" of the Miao City Houdetang Pharmacy Co., Ltd., the cancellation of the enterprise shall stop the drug business activities according to law.
If you aspire to start a commercial growing operation, we have designed the System as a beginning package especially for you to get hands-on experience. This unit is very well thought out, easy to assemble and operate, and includes virtually everything you need to get started (comes with a full year of growing supplies). In weeks, the system will be up to full speed, producing some of the best crops you and your customers will have ever seen.
Greenhouse A Hydroponics,Hydroponic Garden,Hydroponic System
JIANGSU SKYPLAN GREENHOUSE TECHNOLOGY CO.,LTD , https://www.greenhousehydroponic.com